Light, the mesmerizing phenomenon that dazzles our senses, holds a profound significance in understanding the world around us. This article explores the captivating properties of light and its intricate interaction with matter.
The Essence of Light
Light, also known as visible light, encompasses the entire electromagnetic spectrum that can be detected by the human eye. This spectrum spans from long-wavelength radio waves to short-wavelength gamma rays. Defined as fluctuating electric and magnetic fields, light travels at the astounding speed of approximately 300,000 km/s through a vacuum. It can also be described as a stream of photons, which are weightless energy packets that possess both wave-like properties and the ability to travel at the speed of light. The realization that light moves in discrete quanta laid the foundation for the revolutionary Quantum Theory.
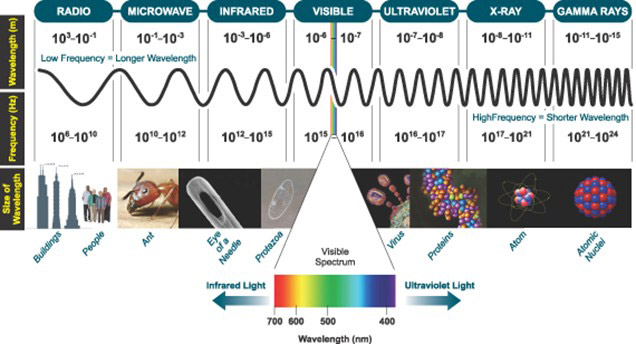
Figure 1: The Electromagnetic Spectrum, highlighting the narrow window of Visible Light that is detectable by the human eye.
Unraveling the Interplay Between Light and Matter
The intricate dance between light and matter holds the key to unlocking the secrets of our universe. Light's interaction with matter can lead to profound transformations, enabling scientists to unravel the properties of various substances. By studying light that originates from or interacts with matter, scientists can delve into the composition of distant stars and galaxies or observe the microscopic processes that occur within living cells.
Read more: Enhance Your Pool with Swimming Pool LED Rope and Strip Lights
Matter, consisting of atoms, ions, or molecules, undergoes interactions with light that give rise to various phenomena. These interactions occur at distinct energy levels associated with the electrons within the matter. Matter may emit light, or photons, or absorb photons in multiple ways.
To represent these energy levels and interactions visually, scientists employ a Jablonski diagram as shown in Figure 2. When matter in its ground state absorbs a photon, it transitions to an excited state with higher energy. Subsequently, the excited state relaxes back to the ground state through internal processes, emitting a photon of lower energy (longer wavelength) than the initial absorbed photon.

Figure 2: Jablonski Diagram example, illustrating transitions between the various energy states of molecules following interaction with a photon.
Read more: How to Conceal LED Strip Lights on Your Ceiling
Illuminating the Path to Knowledge with Spectroscopy
Spectroscopy, a vast field of scientific techniques, enables us to study matter by examining the photons absorbed or emitted during light-matter interactions. By employing spectrographs, which split light into its constituent wavelengths, scientists can unveil the spectral signatures that reveal invaluable information about matter itself. This knowledge empowers us to understand the properties of atoms and molecules, detect the presence of specific materials, and quantify their concentrations within a given sample. Raman spectroscopy, absorption/transmission/reflection spectroscopies, atomic spectroscopy, laser-induced breakdown spectroscopy (LIBS), and transient absorption spectroscopy are among the many techniques through which spectroscopy enriches our scientific understanding.
Read more: Can I Cut My LED Strip Lights? A Comprehensive Guide
Frequently Asked Questions
Q: Can light be detected outside the visible spectrum?
A: Indeed, advanced scientific detectors can perceive a broader range of the electromagnetic spectrum, extending beyond the limits of human vision. Ultraviolet (UV) radiation with wavelengths lower than 400nm and infrared (IR) radiation with wavelengths longer than 700nm lie outside the narrow window of visible light detectable by the human eye.
Q: How does light assist in studying matter?
A: By interacting with matter and producing characteristic energy photons, light provides invaluable insights into the properties and composition of substances. When split into its constituent wavelengths through spectroscopy, this light enables researchers to analyze and understand matter in great detail.
Q: What is the significance of spectroscopy?
A: Spectroscopy plays a pivotal role in scientific research and analysis. It encompasses various techniques that provide valuable information about the composition, properties, and concentrations of atoms and molecules in a sample. From detecting single photons to unveiling ultrafast changes in matter, spectroscopy opens doors to new horizons in scientific exploration.
Q: What are some advanced methods for detecting light?
A: Explore the world of ultrasensitive camera technology capable of detecting single photons. Discover the advanced cameras employed to measure light from distant corners of the universe. Learn about scientific light detectors that can capture ultrafast changes in matter, down to one billionth of a second. Unveil advanced microscopy solutions that directly image rapid processes within living cells, even following the movements of individual molecules.
Let the enchanting world of light captivate your imagination as you embark on a journey through the captivating properties and profound mysteries it holds.

